Home > Insights > Biotech, Pharma Healthcare > What are Advanced Therapies?
What are Advanced Cell Therapies?
By: Kiran Chin
June, 2020

Our Executive Perspectives are informational primers intended for executives and functional leaders. They are intended to provide a calculated mix of technical and commercial understanding of relevant topics in science and technology industries.
What are Advanced Cell Therapies?
“Advanced Therapies” is an emerging field of therapeutics within the biopharma ecosystem that is pushing the ecosystem into new territories. The biopharma world has largely been segmented into two overarching categories: “Large molecule” and “Small molecule”. Small molecules are defined as solid state, oral dose medicines such as Tylenol or Motrin (for example). Large molecule biopharma therapies are biologics that are often administered through injection such as vaccines, monoclonal antibodies and recombinant proteins.
Advanced therapies is an emerging class of “large molecules”. They are biologics but the mechanism of action of these new classes of therapies is different from what has historically been used.
What are Biologics?
Biologics are often classified into vaccines, recombinant proteins and monoclonal antibodies.
A vaccine works by administering the disease-causing bacteria or virus into the body to cause an immune response. The functional outcome of vaccines is that they trigger the body to utilize its natural defenses and remember that response for future exposure.
Recombinant proteins (rProteins) are artificially created proteins using genetic material from human, animal or plant genes. Some common rProteins include growth factors, blood clotting factors, recombinant hormones, and enzymes.
Finally, monoclonal antibodies (mAbs) are antibodies that have been manufactured to generate a specific response within the body. Examples of how mAbs function include deactivating T cells, stimulating red blood cell production and inhibiting TNF (tumor-necrosis factor, a protein that may be over-actively produced in certain disease types such as rheumatoid arthritis).
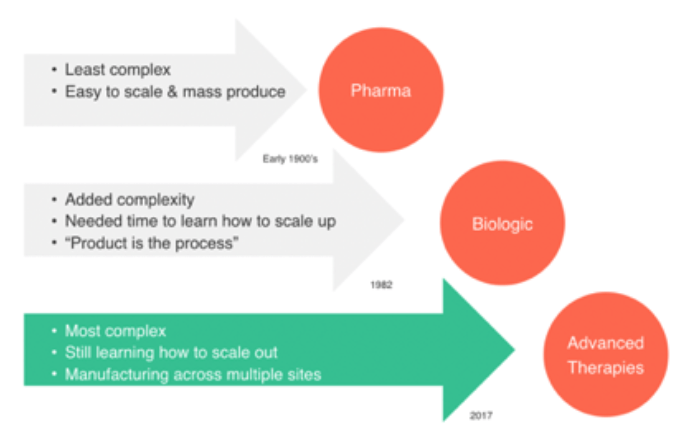
What are Advanced Therapies?
“Advanced therapy” refers to a segment of medical products and treatments that use gene therapy, cell therapy & tissue engineering. Advanced therapy directly targets diseased cells instead of addressing symptoms. There are several types of investigations ongoing within Advanced Therapies that are worth mentioning, including CRISPR, mRNA and weaponized bacteriophage.
Advanced therapies are classified into three key types:
- Gene therapy is a widely used term to refer to the manipulation of genes to affect change on a cellular level – this change seeks to have a direct result on the behavior of cells in the body. The current research in gene therapy has focused on replacing mutated genes, inactivating/silencing mutated genes or inserting new healthy genes to help fight disease. One important point to note within gene therapy is the difference between somatic cell and germline therapy. Somatic cells are found anywhere in the body and genetic mutations caused in somatic cells are not hereditary. Germline therapy involves mutations to the egg or sperm and will be hereditary.
- Tissue-engineering refers to the use of tissues or cells to restore human tissue. Examples include the restoration of a person’s eyesight
- Regenerative medicine refers to the replacement of damaged tissues and organs.
What is Gene Editing?
Gene editing technologies (also called genome editing) give researchers the ability to alter an organism’s DNA by adding, removing or altering locations in the genome. Three emerging gene editing technologies that have generated interest and excitement within the research community include CRISPR-Cas9, short for “clustered regularly interspersed short palindromic repeats”, ZFN, short for “zinc finger nuclease”, and TALEN, short for “transcription activator-like effector nuclease”.
How many cell & gene therapies are in clinical trials?
This number keeps fluctuating – however according to the 2019 annual report issued by the Alliance for Regenerative Medicine (ARM), there were 1060 clinical trials ongoing in various phases.
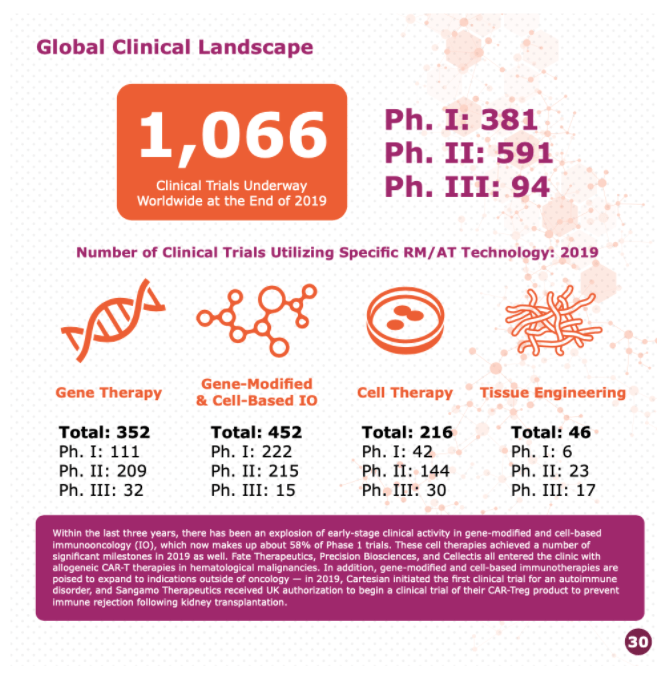
The majority of those clinical trials target oncology as a therapeutic focus (~60% or 618 trials) with the remainder focused loosely on cardio, central nervous, musculoskeletal, metabolic, ophthalmology, dermatology and immunology.
What is the Regulatory Landscape for Advanced Therapies?
Many advanced therapies are still developing and newly emerging into commercial forums, with gene editing at the earliest phase of these therapies. In the United States, the FDA has been working closely with Advanced Therapy companies to identify faster paths to market for emergency therapeutics.
SOURCES
[1] http://www.genetherapynet.com/types-of-gene-therapy.html
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5823056/
[2] https://ct.catapult.org.uk/sites/default/files/publication/DanRabbie_Taking%20Advanced%20Therapy%20Medicinal%20Products%20%28ATMPs%29%20to%20Market_29Jun18.pdf
[3] https://medicine.missouri.edu/centers-institutes-labs/health-ethics/faq/gene-therapy
[4] https://ghr.nlm.nih.gov/primer/therapy/ethics
[5] https://www.nibsc.org/science_and_research/advanced_therapies.aspx
https://www.mayoclinic.org/tests-procedures/bone-marrow-transplant/in-depth/stem-cells/art-20048117?_ga=2.246125441.427798033.1567173794-1353958951.1567173794
[6] https://www.cellectis.com/en/research/talen
[7] https://www.bccresearch.com/market-research/biotechnology/genetic-modification-therapies-clinical-applications-report.html
[8] https://lifescience.roche.com/en_us/articles/mrna-silencing-by-rna-interference.html
[9] https://labiotech.eu/infographics/rnai-infographic/
[10] https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
Connect with our experts today